2e-
Copper Electrolysis Collection
Project Background
As we observed our environment we realized that they are two main types of forms that surround us, those, which are man-made and natural ones. We lose track of this context, maybe because of our life rhythm, or maybe because we are too immersed in our man-made world. What is fascinating about nature, is that is ever-changing.
2e- focuses on appropriating our natural environment by analyzing it. I observed change, specifically a concept called Entropy which sometimes is defined as a gradual decline of arranged elements into disorder. Through this work, I focus on developing a set of conditions that will affect and create forms, in other words, I allow the form to manifest itself.
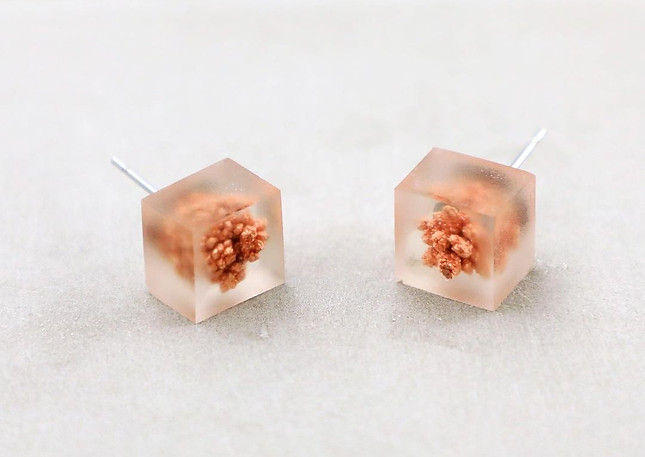
I spent months testing electrolysis metal crystals. These pieces have a special way of capturing nature in order to freeze the original sparkle of the metal crystals. The basic principle of crystal growing is to electrolyze a solution of sulfate with two copper electrodes. In this process, the anode (+) acts as a donor, and the cathode (-) serves as a receiver. The copper molecules are separated from the scrap copper (+) and aggregated on the negative copper part which gradually forms coral-like copper crystals.

Entropy: a gradual decline of arranged elements into disorder

Material Experiment: Copper Electrolysis

We collected scrap coppers and converted them into copper crystal jewelry through electrolysis. The basic principle of crystal growing is to electrolyze a solution of sulfate with two copper electrodes. In this process, the anode (+) acts as a donor, and the cathode (-) acts as a receiver. The copper molecules are separated from the scrap copper (+) and aggregated on the negative copper part which gradually forms coral-like copper crystals.
Experimenting and Learning


The current and voltage are proportional to the electrolysis forming time and inversely proportional to the strength of the finished product.
The distance between the positive and negative electrodes is inversely proportional to the reaction time and is proportional to the strength of the finished product.
Copper molecules tend to grow concentrated on the higher curvature of the arc.
Copper molecules tend to grow concentrated on the sharper corners and edges.

The direction of copper crystal growth is determined by the relative position of the positive electrode.

Saturated copper sulfate solution is a prerequisite for the growth of copper crystals.

We spend months experimenting with the copper electrolysis reaction and exploring the diversity of copper entropy form which is influenced by the anode, cathode, voltage, and current. With these principles we found, we created a series of copper from samples in 2017 and developed it wearable in 2018.
Design Outcome